
Analysis of the American Rheumatology Network-Trio Health (ARN-Trio) registry finds a surge in new prescriptions for Merck’s Renflexis infliximab biosimilar. The ARN-Trio registry is the first rheumatology registry to combine pharmacy dispensing data with electronic medical records (EMR) to provide unparalleled real-world insights. ARN-Trio is the largest registry of biosimilar use in community rheumatology practice, and data from 5 large practices chosen for geographic variety reveal insightful trends in infliximab biosimilar use.
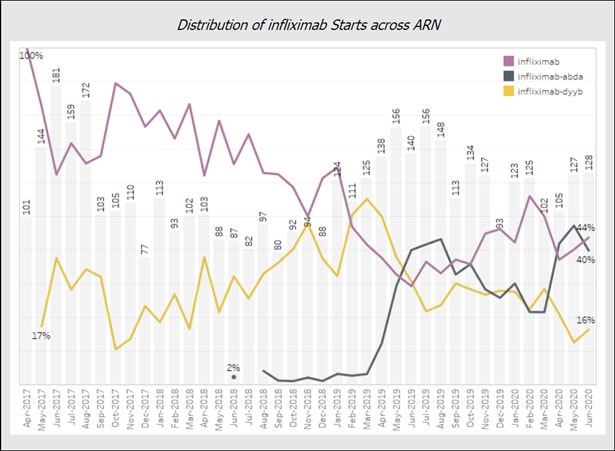
Initially, the use of Pfizer’s Inflectra (infliximab-dyyb) followed by Merck’s Reflexis (infliximab abda) followed trajectories largely explained by the well-documented formulary restrictions and time to market challenges inherent to biosimilar adoption in the U.S pharmaceutical ecology. However, recent data reveals a steep rise in Renflexis starts at the expense of Inflectra market share.
Ray Waldrup, CEO of ARN, offers a likely explanation, “Clinician comfort with biosimilars is no longer the driving factor for use of these medications, we are now entering into an era where scrutiny of secondary factors such as manufacturer support of patients and practices is paramount.” When asked what might specifically be driving the surge in Merck’s Renflexis recently, Waldrup notes, “Merck has shown a commitment to patients and practices, particularly their willingness to support practices as they perform frequent and timely benefit verification for these medications. Verifying benefits ensures patient access by avoiding unexpected costs and protects practices from unpaid claims. When considering the concerns of community rheumatologists, Merck ‘gets it’ and this is what makes them a good partner.”
ARN remains committed to the appropriate adoption of biosimilars as an effective way to reduce medication costs, and thereby improve patient access to these life-altering therapies. ARN Executive Chairman, Colin Edgerton, MD, reflects, “Biosimilars have now proven their potential to reduce medication prices, as we see with the infliximab market. ARN stands with the rheumatology community in appropriate adoption of biosimilars, and I would highlight the American College of Rheumatology position paper on biosimilars as an excellent review of where we stand.”

